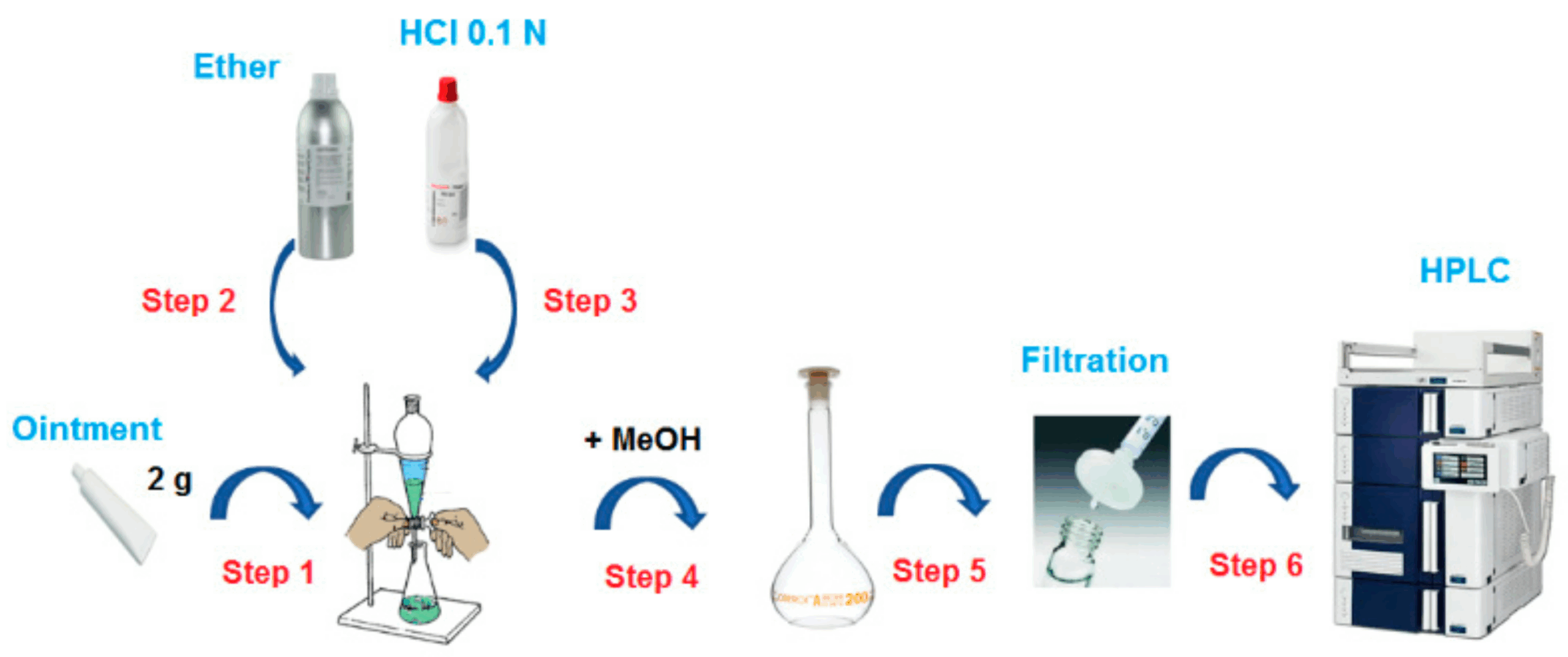
Method Development and Validation Services
Our Method Development and Validation services deliver robust, compliant analytical solutions to support the entire lifecycle of pharmaceutical, nutraceutical, and chemical products. We design, optimize, and validate analytical methods that are scientifically sound, regulatory-compliant, and tailored to the unique properties of your materials and formulations.
Each method is developed in full alignment with ICH, USP, EP, and BP guidelines, ensuring suitability for its intended use and readiness for application in GMP environments. From early development to commercial manufacturing and release, our team provides dependable analytical tools that safeguard product quality, consistency, and regulatory success.
Core Capabilities
Assay & Related Substances (RS)
-
Development of stability-indicating methods for APIs and finished products
-
Quantification of active ingredients, impurities, and degradation products
-
Optimization for selectivity, accuracy, and robustness
-
Application of chromatographic, spectroscopic, and wet chemistry techniques
Dissolution Method Development
-
Design and optimization of discriminating dissolution methods
-
Evaluation of critical parameters (medium composition, pH, agitation, apparatus type)
-
Establishment of meaningful specifications and performance criteria
-
Conduct of profiling and comparison studies to support formulation and bioequivalence assessments
Other Analytical Method Development Areas
-
Identification and characterization of impurities and degradation pathways
-
Cleaning validation and residue analysis methods
-
Physical and chemical testing (pH, appearance, viscosity, moisture, etc.)
-
Use of spectroscopic (UV-Vis, FTIR) and chromatographic (HPLC, GC) techniques
-
Adaptation or remediation of compendial methods for specific formulations
Method Validation Services
-
Conducted in accordance with ICH Q2(R2) and other global regulatory standards
-
Validation of accuracy, precision, linearity, range, robustness, specificity, and detection limits
-
Development and execution of validation protocols and summary reports
-
Transfer and verification of validated methods between laboratories or sites
-
Dissolution discriminatory analysis and assurance